Nothing better the home: Decentralized Clinical Trial
A Decentralized Clinical Trial (DCT) is a type of clinical trial that leverages technology and innovative approaches to conduct various aspects of the trial in a decentralized or remote manner. Traditional clinical trials often require participants to visit physical sites, such as hospitals or research centers, for activities like study visits, data collection, and monitoring. In contrast, DCTs aim to reduce the need for participants to travel and increase the flexibility of trial operations.
Key features of decentralized clinical trials include:
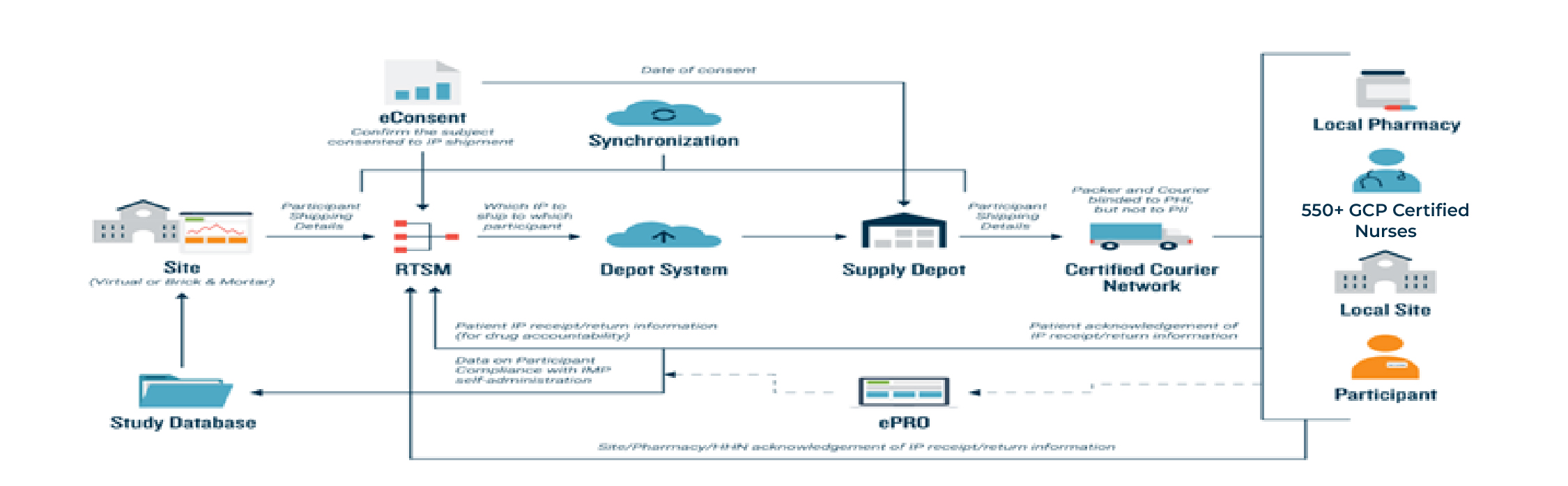
1. Remote Participation: DCTs enable participants to engage in the trial from their own homes or local healthcare facilities. Nurses play a pivotal role in providing IP services and healthcare, minimizing the need for travel and improving accessibility for a wider range of participants.
2. Remote Monitoring: Participant data can be collected remotely using wearable devices, mobile apps, and telehealth technologies. This allows researchers to continuously monitor vital signs, patient-reported outcomes, and other relevant data without requiring physical visits.
3. Telemedicine: Virtual visits and telemedicine consultations are employed for interactions between participants and study staff, including medical assessments and check-ins.
4. Wearable Devices and Sensors: Wearable devices can monitor various physiological parameters, providing real-time data for researchers. These devices can track metrics like heart rate, activity levels, and sleep patterns.
5. Decentralized Investigator Sites: In some DCTs, investigator sites might be located in various healthcare facilities rather than centralized at a single location, making it more convenient for participants.
Benefits of Decentralized Clinical Trials:
1. Access: DCTs can reach a more diverse participant population by removing geographical barriers and enabling those who might have difficulty traveling to participate.
2. Convenience: Participants have greater flexibility since they do not need to travel to specific sites for every visit, making participation more convenient.
3. Real-world Data: DCTs can capture more real-world data by monitoring participants in their everyday environments, potentially leading to more relevant and accurate outcomes.
4. Cost and Time Efficiency: DCTs can reduce trial costs associated with site operations, monitoring, and participant compensation. They can also speed up recruitment and data collection.
5. Patient-Centric Approach: DCTs prioritize the convenience and comfort of participants, potentially leading to increased engagement and retention.
Challenges and Considerations:
1. Regulatory and Ethical Considerations: Regulatory bodies must adapt to new trial models, and ethical considerations related to participant privacy and data security must be addressed.
2. Technology Barriers: Participants and researchers need access to appropriate technology, which could pose barriers for some populations.
3. Data Integrity: Ensuring the integrity and quality of remotely collected data is essential for maintaining the credibility of trial results.
4. Participant Engagement: Maintaining participant engagement and adherence to trial protocols without regular face-to-face interactions can be a challenge.
Decentralized clinical trials represent a significant shift in how clinical research is conducted, offering the potential to improve trial efficiency, participant experience, and data quality. However, successful implementation requires careful planning, technological infrastructure, regulatory alignment, and consideration of the specific trial's goals and population

